regulatory
Leading the Cardiovascular Imaging Industry
Circle Improves patient outcome by revolutionizing the cardiovascular healthcare industry through innovation, and enabling healthcare providers to complete effective and precise analysis.
Clearances

United States

Canada

Australia

EU CE Mark
Malaysia

Singapore

Japan
Taiwan

South Korea

India

El Salvador

China
cvi42
NMPA Approved

Brazil

United Arab Emirates

Thailand

Indonesia

Argentina

Saudi Arabia

Colombia

Chile

South Africa

Israel
cvi42
25830055
StrokeSENS
25830062
WATCHMAN TruPlan Clearances
Access information related to WATCHMAN TruPlan, including user manuals and clearances.
Quality Policy
Circle endeavors to constantly innovate and improve our cardiovascular and neurovascular imaging software by clearly understanding the ever-changing needs and requirements of physicians worldwide. We strive to serve them with clinical tools that provide for the best possible outcomes.
Through adherence to and improvement of our Quality Management System we continuously strive to be the leader in our industry, providing value added products for healthcare institutions worldwide. We are guided by our core values of quality, innovation, people, and customers.
To achieve this Circle will:
- Ensure that our product and quality management system is effective and in compliance with the applicable standards and regulations;
- Ensure that the quality objectives are established and reviewed;
- Ensure that the products we design, develop, and manufacture meet their stated or implied specifications;
- Understand the requirements of our customers and address their relevant concerns; and
- Involve employees at all levels of the organization.
Environmental, Social, and Governance
Circle Cardiovascular Imaging, as a Software as a Medical Device (SaMD) company that operates in many countries globally, strives to create environments that enhance the lives of our customers and employees, but a changing world brings both uncertainty and opportunities.
Compliance Information
Circle Cardiovascular Imaging Inc. holds globally recognized ISO 13485:2016 and MDSAP certificates from the British Standards Institution (BSI). In addition to ISO 13485:2016 and MDSAP, the company quality system is established and maintained in alignment with global medical device regulations, including European Medical Device Regulation (EU) 2017/745, Australian Therapeutic Goods (Medical Devices) Regulations, U.S. FDA Quality Management System Regulation (QMSR) - 21 CFR Part 820. Circle continues to monitor regulatory developments and updates its quality system as required to ensure ongoing compliance with evolving global regulatory requirements.
Circle’s software development process incorporates several internationally recognized standards and guidance documents, including: BS EN (IEC) 62304:2006+A1:2015, BS EN (IEC) 62366-1:2015+A1:2020, and IEC 82304-1:2016. An intensive risk management process, based on BS EN (ISO) 14971:2019+A11:2021, is also implemented as part of the development process, to capture the potential harms, hazards, and hazardous situations, and to mitigate these identified risks.
If you require further information on Circle's Quality System or the regulatory approval status for Circle products, please contact the following:
Dr. Shirantha Samarappuli
Vice President
Regulatory Affairs and QMS
Email: shirantha.samarappuli@circlecvi.com
Phone: +1 (587) 747-4692
BSI ISO 13485:2016 Certificate # FM 539204 (Effective: Aug 31st, 2024)
BSI ISO 13485:2016 Certificate # MDSAP 689664 (Effective: Aug 31st, 2024)
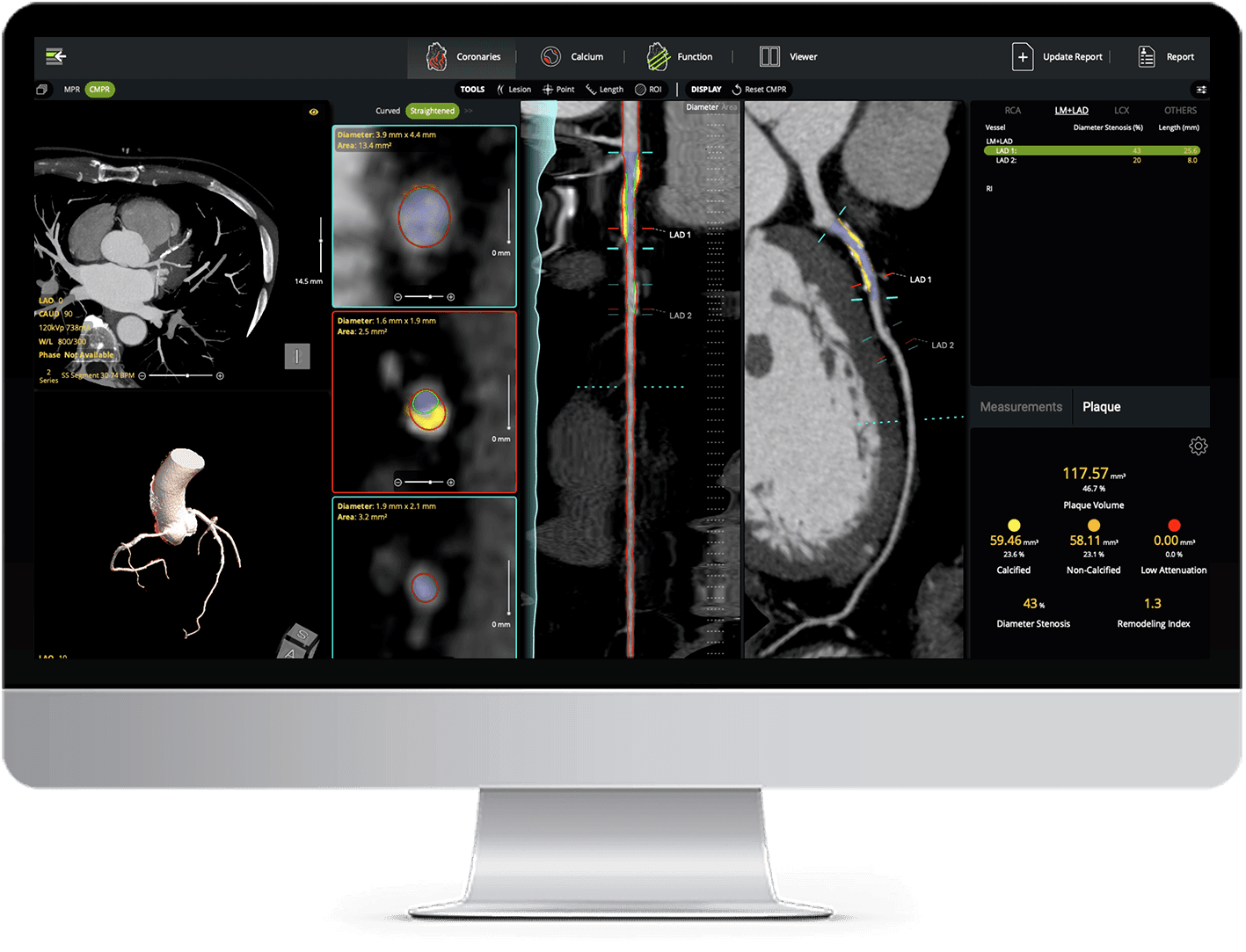
AI Plaque Analysis
AI-powered coronary plaque quantification with detailed reporting - fully interactive and integrated into your CT workflow.
